Compared to other forms of PAP therapy, ASV offers significant benefits for the treatment of central SDB* – including improvement of AHI, reduction of respiratory events and alleviation of daytime sleepiness.
Any patient with Left Ventricular Ejection Fraction (LVEF) >45% is eligible for ASV.1,2,3,4
We can confirm that the observed mortality risk in the SERVE-HF study occurs in patients with LVEF ≤45% and that the harmful effects of ASV correlate with pre-existing LV systolic impairment.5
Reduced LVEF should be excluded before starting ASV.1 Before using ASV, it is important to ensure that LVEF is > 45%. Echocardiography is recommended for this purpose.
Experts statements1,2,3,4 and healthcare authorities agree that patients with LVEF >45% remain eligible for ASV when there is a clinical rationale for using it. ASV is eligible in these different situations: 1,2,3,4
Since May 2015, the French and German healthcare authorities have agreed to limit the contra-indication to heart failure with reduced EF ≤45%.4
Find out more about the ResMed device that delivers ASV therapy: AirCurve 10 CS PaceWave
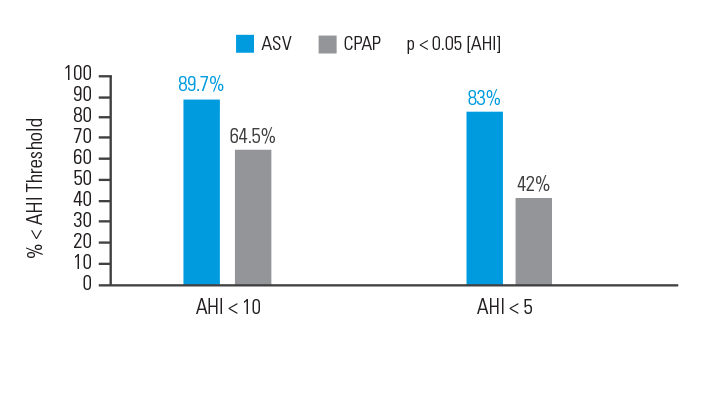
In an intention-to-treat analysis, success (apnoea hypopnoea index [AHI] < 10) at 90 days of therapy was achieved in 89.7% of patients treated with ASV versus 64.5% of participants treated with CPAP.6
[N = 66, prospective randomised trial]
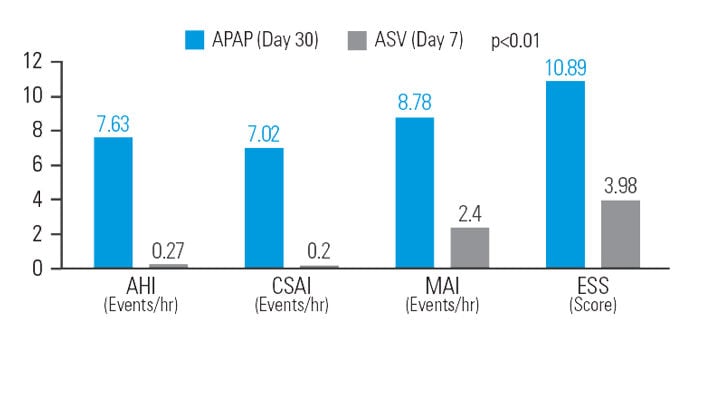
After 30 days of APAP treatment, ASV provided a further reduction (compared to baseline) of 12.9% in AHI, 48.5% in central sleep apnoea index (CSAI), 26.1% in micro-arousal index (MAI), and 37.9% in Epworth Sleepiness Scale (ESS) score at similar mean pressure.7
[N = 42, sequential study]
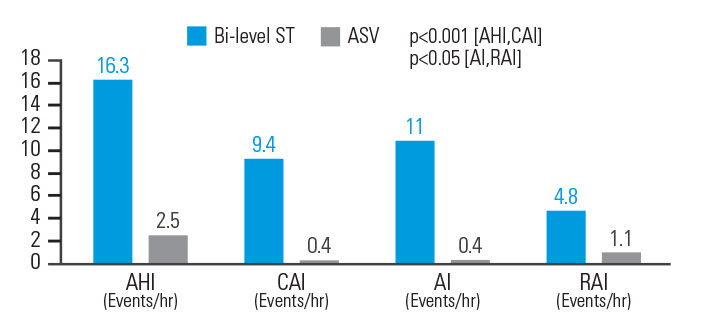
In opioid-induced CSA, ASV therapy reduced AHI by 84.7%, central apnoea index (CAI) by 95.7%, Apnoea Index (AI) by 96.4%, and respiratory arousal index (RAI) by 77.1% when compared to bi-level ST. Respiratory parameters were normalised in 83.3% of patients on ASVAuto but only 33.3% of patients on bi-level ST.8
[N = 18, prospective, randomised crossover polysomnography study]
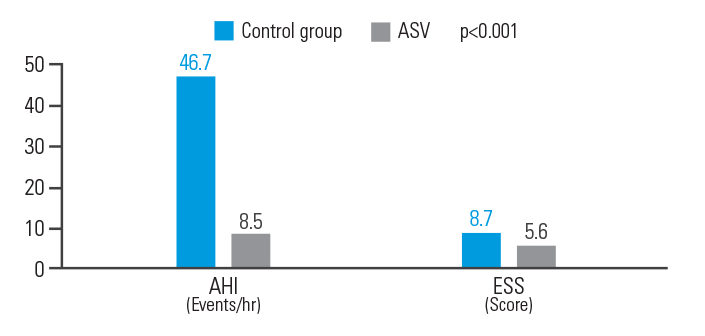
ASV therapy improved outcomes for post-acute ischemic stroke patients with CSA, reducing AHI by 81.8% and ESS by 35.6%.9
[N = 15, single centre retrospective analysis]
Prospective randomised and observational heart failure studies (already presented and shortly to be published) have suggested that ASV may be beneficial in heart failure patients with preserved ejection fraction and in those who have CSA with coexisting obstructive sleep apnoea.10 Currently there is no evidence that these patients would be at any risk of harm from ASV therapy.